Wednesday, August 19, 2015
Thursday, June 4, 2015
End of year survey
Please take some time to thoughtfully reply to the end of the year survey. This will provide feedback that should help me improve the course next year. Thank you.
End of year survey
End of year survey
Wednesday, June 3, 2015
Qualitative Analysis and Final Exam Unit 9
For the last few weeks of school we will be working on qualitative analysis of unknown solutions. This extended lab will summarize many of the reaction types we have studied this year and will increase student analytical skills.
The final exam will be comprehensive. Students should review all notes from the year, study chapter reviews and complete a practice exam during the last days before the final exam.
Online
Richard Thornley IB review videos
Review only qualitative analysis, atomic structure, periodicity, bonding, equilibrium and acids/bases videos for the chem year one final.
The final exam will be comprehensive. Students should review all notes from the year, study chapter reviews and complete a practice exam during the last days before the final exam.
Online
Richard Thornley IB review videos
Review only qualitative analysis, atomic structure, periodicity, bonding, equilibrium and acids/bases videos for the chem year one final.
Thursday, April 30, 2015
Acids and Bases Unit 8
This unit is on caustics. Study all of Chapter 15 and complete chapter review problems 1 to 31 odd and all of Chapter 16 and complete chapter review problems 1 to 25 odd. The unit quiz will be on 5/26
DUE DATES
Worksheet 15.1: Naming acids, conjugate acids and bases due 5/7
Worksheet 1: Calculating pH due 5/26
Worksheet 2: pH and pOH due 5/26
Lab 2 : Titrations: Data Calculations Conclusion due 5/7
Lab 3 : Titrations: Unknown concentration determination due 5/16
Lab 4 : Determining Hydrogen Ion Concentration of Strong and Weak Acids due 5/22
Alternative assignment second semester due 5/29
Chemistry Acids and Bases worksheet for the quiz ANSWER sheet
Acid Bases ENDMEMO
ONLINE
Conjugate acids and bases brightstorm
Acid base reactions in solution crashcourse
Strength of acids and bases brightstorm
pH and pOH crashcourse
pH, pOH, pKw khan academy
Setting up and performing a titration
Titration calculations
Universal indicator demo
Cheeseburger in HCl
Coke cans in HCl and NaOH
Goose skull in HCl
TESLA: Master of Lightening.
DUE DATES
Worksheet 15.1: Naming acids, conjugate acids and bases due 5/7
Worksheet 1: Calculating pH due 5/26
Worksheet 2: pH and pOH due 5/26
Lab 2 : Titrations: Data Calculations Conclusion due 5/7
Lab 3 : Titrations: Unknown concentration determination due 5/16
Lab 4 : Determining Hydrogen Ion Concentration of Strong and Weak Acids due 5/22
Alternative assignment second semester due 5/29
Chemistry Acids and Bases worksheet for the quiz ANSWER sheet
Acid Bases ENDMEMO
ONLINE
Conjugate acids and bases brightstorm
Acid base reactions in solution crashcourse
Strength of acids and bases brightstorm
pH and pOH crashcourse
pH, pOH, pKw khan academy
Setting up and performing a titration
Titration calculations
Universal indicator demo
Cheeseburger in HCl
Coke cans in HCl and NaOH
Goose skull in HCl
TESLA: Master of Lightening.
Thursday, April 9, 2015
Equilibrium Unit 7
This unit is on equilibrium. In chapter 12 study section 12-3 only p. 372 to 382. Complete chapter 12 review problems 8 to 15 all. In chapter 18 study the whole chapter p. 553 to 584 except skip section 18-3 and complete 1 to 37 odd review problems. There will be a test on equilibrium on 4/30.
In addition to study of the text and your class notes you should consider the internet, especially YouTube chemistry channels, an essential part of learning chemistry well. WATCH THE VIDEOS... you will slowly build important concepts...
DUE DATES
Lab 10.1 Determining the Density of Carbon Dioxide is due 4/13.
Equilibrium simulation lab (3 labeled graphs done on excel)
Worksheet 1: Dynamic Equilibrium/Le Chatelier's principle due 4/21
Lab E 1(34): Chemical Equilibrium and Le Chatelier's 4/27
Worksheet 2: Equilibrium constant/calculations due 4/23
Lab E 2(35): Solubility Product Constant due 4/29
Worksheet 3: Solubility Product Constant/Le Chatelier's due 4/30
Alternative assignment second semester
ONLINE
Equilibrium Thornley IB
Equilibrium Crashcourse
Chemical equilibrium OSU chem
Le Chatelier's principal Khan academy
Le Chatelier's principal Bozeman science
About Fritz Haber
The dawn of chemical warfare
Industrial production of ammonia
Haber Bosch process
Water and solutions Crashcourse
Solubility equilibrium Brightstorm
Solubility product constant table 25 C
Tracking pollution chemists celebrate earth day
In addition to study of the text and your class notes you should consider the internet, especially YouTube chemistry channels, an essential part of learning chemistry well. WATCH THE VIDEOS... you will slowly build important concepts...
DUE DATES
Lab 10.1 Determining the Density of Carbon Dioxide is due 4/13.
Equilibrium simulation lab (3 labeled graphs done on excel)
Worksheet 1: Dynamic Equilibrium/Le Chatelier's principle due 4/21
Lab E 1(34): Chemical Equilibrium and Le Chatelier's 4/27
Worksheet 2: Equilibrium constant/calculations due 4/23
Lab E 2(35): Solubility Product Constant due 4/29
Worksheet 3: Solubility Product Constant/Le Chatelier's due 4/30
Alternative assignment second semester
ONLINE
Equilibrium Thornley IB
Equilibrium Crashcourse
Chemical equilibrium OSU chem
Le Chatelier's principal Khan academy
Le Chatelier's principal Bozeman science
About Fritz Haber
The dawn of chemical warfare
Industrial production of ammonia
Haber Bosch process
Water and solutions Crashcourse
Solubility equilibrium Brightstorm
Solubility product constant table 25 C
Tracking pollution chemists celebrate earth day
Monday, March 9, 2015
Gases Unit 6
This unit is on Gases. Topics include the ideal gas law (PV = nRT), the combined gas law, Dalton's, Boyle's, Charles's, Gay-Lussac's, and Avogadro's gas laws. Study Chapter 10 and complete chapter review problems 1 to 33 odd and Chapter 11 (sections 11-1 to 11-3 only) and complete chapter review problems 1 to 29 odd by 4/6. We will have a quiz on Monday 4/6.
In addition to study of the text and your class notes you should consider the internet, especially YouTube chemistry channels, an essential part of learning chemistry well. WATCH THE VIDEOS... you will slowly build important concepts...
DUE DATES
HYDRATE ANALYSIS full write up is due 3/9
Acetylene combustion ratio lab due 3/12
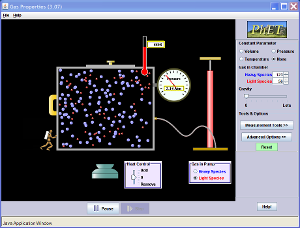
Gas Properties (PhET SIM) handout + assigned graph due 3/20
Worksheet 10.1 Boyle's Law due 3/20
Worksheet 10.2 Ideal Gas Law due 4/2
Lab 10.2 Mass-Volume Relationships in Reactions due 4/2
Lab Determining the Density of Carbon Dioxide due 4/13
ONLINE
Ideal Gas Law Crashcourse
Stoichiometry in combustion of acetylene
The race for absolute zero nova documentary
The conquest of cold nova documentary
In addition to study of the text and your class notes you should consider the internet, especially YouTube chemistry channels, an essential part of learning chemistry well. WATCH THE VIDEOS... you will slowly build important concepts...
DUE DATES
HYDRATE ANALYSIS full write up is due 3/9
Acetylene combustion ratio lab due 3/12
Gas Properties (PhET SIM) handout + assigned graph due 3/20
Worksheet 10.1 Boyle's Law due 3/20
Worksheet 10.2 Ideal Gas Law due 4/2
Lab 10.2 Mass-Volume Relationships in Reactions due 4/2
Lab Determining the Density of Carbon Dioxide due 4/13
ONLINE
Ideal Gas Law Crashcourse
Stoichiometry in combustion of acetylene
The race for absolute zero nova documentary
The conquest of cold nova documentary
Torricelli barometer
Gas Laws TedEd
Gas Laws 2 TedEd
Boyle's law brightstorm
Combined gas law stoichiometry brightstorm
Ideal gas law stoichiometry brightstorm
Ideal gas law example problem Khan academy
Solid Nitrogen
Additional directions for gas properties SIM
An excel tutorial
Chemix app for diagramming apparatus
Gas Laws TedEd
Gas Laws 2 TedEd
Boyle's law brightstorm
Combined gas law stoichiometry brightstorm
Ideal gas law stoichiometry brightstorm
Ideal gas law example problem Khan academy
Solid Nitrogen
Additional directions for gas properties SIM
An excel tutorial
Chemix app for diagramming apparatus
Tuesday, March 3, 2015
Hydrate analysis
Portland Public Schools Common Science Assignment -Chemistry
Design, implement and analyze the results of an experiment that determines the percentage of water in a hydrated solid.
Complete a full lab write up with the following sections...
1) Title
2) Purpose
3) Proceedure
4) 'Raw' data
5) Processed data and calculations
6) Conclusion with sources of error and suggestions for improvement
DUE 3/9
Hydrate analysis all class data
To make apparatus diagram use this chemix app
How to use chemix for apparatus diagrams youtube video
Design, implement and analyze the results of an experiment that determines the percentage of water in a hydrated solid.
Complete a full lab write up with the following sections...
1) Title
2) Purpose
3) Proceedure
4) 'Raw' data
5) Processed data and calculations
6) Conclusion with sources of error and suggestions for improvement
DUE 3/9
Hydrate analysis all class data
To make apparatus diagram use this chemix app
How to use chemix for apparatus diagrams youtube video
Friday, January 16, 2015
Stoichiometry Unit 5
This unit is on stoichiometry. You will be studying chemical formulas, reactions, equations and the mole concept. There will also be some work done learning mass and gaseous volume relationships; the ideal gas equation. Chapter 8 complete questions 1 to 35 odd. Chapter 9 complete questions 1 to 29 odd. There are many online videos that can help you learn stoichiometry. Outside of what we learn in class, I suggest using khan academy or brightstorm to 'pick it up the first time' and reviewing with the IB style thornley videos or crashcourse... see links below. Continue to work on memorizing ions from the previous unit if you have not learned them all yet. You will use them throughout the rest of the year. Keep memorizing ions! Test on 2/26
In addition to study of the text and your class notes you should consider the internet, especially YouTube chemistry channels, an essential part of learning chemistry well. WATCH THE VIDEOS... you will slowly build important concepts...
Students who are not prepared in math skills are the students who struggle the most and have to spend the most time on chemistry. If you are not concurrently enrolled in algebra 3-4 or above you may not really have the prerequisite math ability for chemistry. Students in geometry will need to spend more time on problem solving parts of chemistry, especially in the second semester, to keep up.
DUE DATES
Lab 8.1: Types of chemical reactions due 2/5
Worksheet 9.1: the mole/stoichiometry 1 due 2/12
Lab 8.2: Relating Moles to coefficients of a chemical equation due 2/12
Worksheet 9.2 Mass/Mass stoichiometry due 2/23
Lab 9.1 Mole and Mass relationships due 2/23
Lab 9.2 Mass-Mass Relationships due 3/2
Chapter review problems due by the day of the test 2/26
ONLINE
Balancing chemical equations Khan academy
Precipitation Reactions crash course
Beautiful chemistry website. Awesome.
IB style 'the mole' stoichiometry thornley
IB 'number of particles' stoichiometry thornley
IB 'relative atomic mass/molecular mass' thornley
IB 'mass of a mole' thornley
IB 'mole problems' thornley
stoichiometry crash course
stoichiometry brightstorm
stoichiometry khan academy
limewater test for carbon dioxide
lead iodide class data set period 5
Period 1 Lab 8.2 spreadsheet
Period 2 Lab 8.2 spreadsheet
Period 4 Lab 8.2 spreadsheet
Period 5 Lab 8.2 spreadsheet
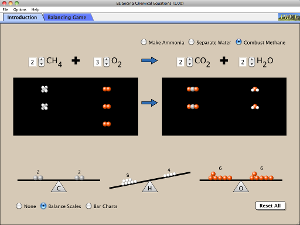
pHet simulation balancing chemical equations handout
In addition to study of the text and your class notes you should consider the internet, especially YouTube chemistry channels, an essential part of learning chemistry well. WATCH THE VIDEOS... you will slowly build important concepts...
Students who are not prepared in math skills are the students who struggle the most and have to spend the most time on chemistry. If you are not concurrently enrolled in algebra 3-4 or above you may not really have the prerequisite math ability for chemistry. Students in geometry will need to spend more time on problem solving parts of chemistry, especially in the second semester, to keep up.
DUE DATES
Lab 8.1: Types of chemical reactions due 2/5
Worksheet 9.1: the mole/stoichiometry 1 due 2/12
Lab 8.2: Relating Moles to coefficients of a chemical equation due 2/12
Worksheet 9.2 Mass/Mass stoichiometry due 2/23
Lab 9.1 Mole and Mass relationships due 2/23
Lab 9.2 Mass-Mass Relationships due 3/2
Chapter review problems due by the day of the test 2/26
ONLINE
Balancing chemical equations Khan academy
Precipitation Reactions crash course
Beautiful chemistry website. Awesome.
IB style 'the mole' stoichiometry thornley
IB 'number of particles' stoichiometry thornley
IB 'relative atomic mass/molecular mass' thornley
IB 'mass of a mole' thornley
IB 'mole problems' thornley
stoichiometry crash course
stoichiometry brightstorm
stoichiometry khan academy
limewater test for carbon dioxide
lead iodide class data set period 5
Period 1 Lab 8.2 spreadsheet
Period 2 Lab 8.2 spreadsheet
Period 4 Lab 8.2 spreadsheet
Period 5 Lab 8.2 spreadsheet
pHet simulation balancing chemical equations handout
Subscribe to:
Posts (Atom)